(9 December 2022)
Effect of Bleaching Solution pH
The point of this whole page is to elucidate just how the process of bleaching mature Cyp seeds stimulates their germination. That extended bleaching of Cyp seeds in hypochlorite bleach greatly improves germination was discovered independently by Van Waes and Debergh (1986) in Belgium and Anderson (1989) in Canada, and I have been using such a treatment ever since I began propagating Cyps in 1989. In this context, "extended" means bleaching for longer than is necessary simply for surface sterilization of the seed. For about as long as I have used the bleaching treatment to improve the germination of mature Cyp seeds, I have wondered about the mechanisms involved in its effectiveness. In addition to surface sterilizing the seed to remove foreign organisms that would infect the cultures, the bleaching process serves to accelerate germination and to increase percentage germination, but how?
Early on I wondered whether the high pH of my bleaching solutions had something to do with improving germination. In 2000-1 I had been working with seed from a C. pubescens capsule that had been collected the previous fall, and found that this particular seed required an unusually long period in the bleach to germinate well. With this seed germination was excellent after soaking in a 10% solution of commercial Clorox (6% NaOCl) for 98 minutes in cultures sown 31 December 2000. The pH of this solution was 12.3. Did the bleaching solution act through the action of oxidizing germination inhibitors by free chlorine in the solution, or did the high pH of the solution somehow remove or inactivate them?
As a first experiment to see whether the high pH of the bleaching solution played a role in the process, I made a trial in which I soaked the seed for 82 minutes in a solution of NaOH with no NaOCl adjusted to pH 12.3. This soaking was followed by pouring off the NaOH and replacing it with 10% Clorox (0.6% NaOCl) bleaching solution for 16 minutes to effect surface sterilization before sowing. Thus the seed was again subjected to a pH 12.3 solution for a total 98 minutes. Germination of the seed after this alternate treatment was also excellent; I estimated it at roughly 70%. Thus bleaching for 98 minutes could be replaced by soaking in a pH 12.3 NaOH solution for 82 minutes followed by short bleaching, just long enough to surface sterilize the seed.
An elementary chemistry calculation shows that the concentration of NaOH or KOH that has pH 12.3 is 0.02 N, and subsequently I did trials with soaking mature seeds of several other Cyp species to see if this pretreatment could shorten bleaching time. The presoak was effective to varying degrees; there was appreciable shortening in the bleaching time for C. candidum, makasin, pubescens, and especially macranthos, but hardly any effect in C. arietinum. I further found that soaking in 0.02 N NaOH or KOH had no deleterious effect on the seeds, and so I now routinely soak seeds in 0.02 N KOH for several hours before bleaching. My choice of KOH over NaOH for routine soaking is primarily philosophical; I may as well employ a base with a cation plants can use as opposed to the Na, which they cannot.
Conceptual Model of Bleaching
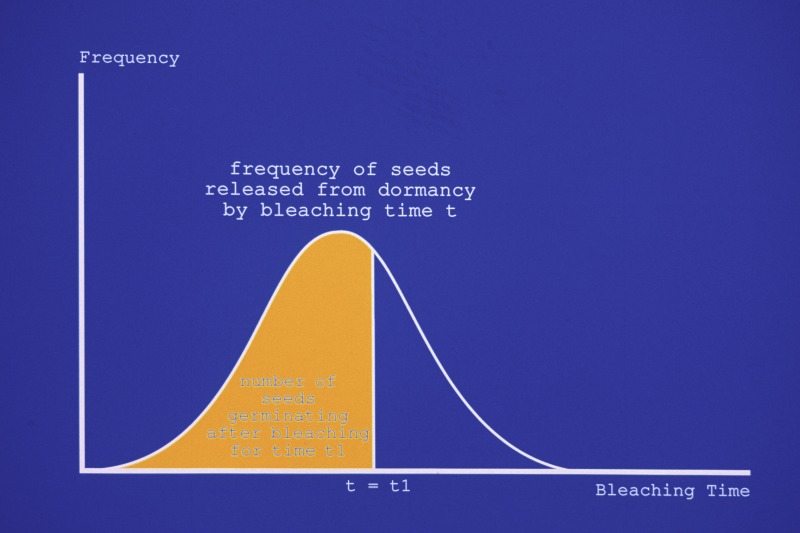
Since the early 1990s, I've held a simple conceptual model of what happens during the bleaching process. In this model, I imagine that the percentage or the frequency of seeds germinating after a given time in the bleaching solution follows a normal or Gaussian distribution as shown at the right. In this figure, the number of seeds germinating after bleaching time t1 is depicted as the yellow shaded area under the curve. I have assumed a normal distribution because so many biological parameters such as the weight or height of an organism follow this distribution. The mathematical explanation for the common occurrence of the normal distribution in biology is that a given biological parameter results from the sum of many variables so that the normality of the distribution of the parameter follows from the Law of Large Numbers. Thus I am merely assuming that the degree of bleaching required to release a seed from dormancy results from the contributions of many variables including genetics and mother plant environment. The exact form of the actual distribution is not that important to my model, and I have chosen the normal distribution not only because it may be realized in nature but also because it is easy to draw.
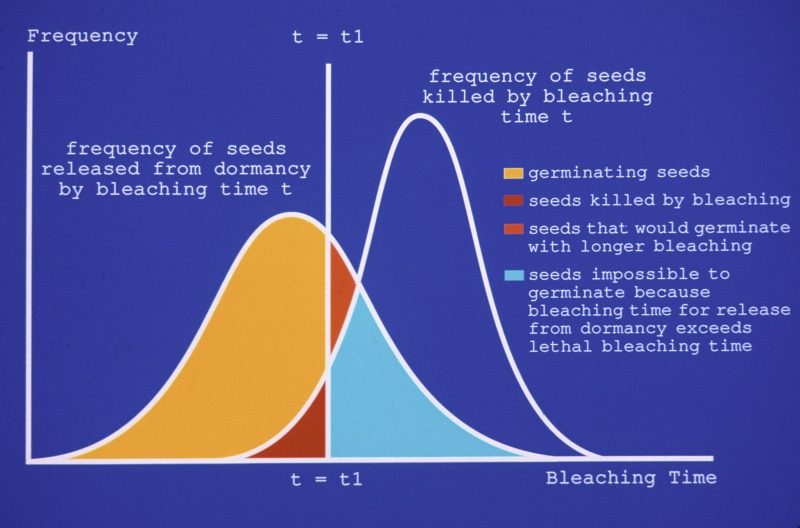
Bleaching unfortunately also kills seeds, that is, seeds bleached too long will never germinate. Consequently the time in the bleaching solution must be chosen so as to be long enough to promote germination, but not so long as to kill the embryo. In my model, I also assume that the time required to kill a seed follows a normal distribution, and the biological argument for that distribution is the same as above. Thus the frequency of death of seeds during the bleaching can be depicted as below:

The results of bleaching can be obtained as a superposition of these two distributions as shown at the bottom of this section. The intersection of the curves for these two normal distributions defines areas that characterize the fate of the seeds being bleached. In the figure, colors designate these different outcomes. Yellow represents the seeds that are released from dormancy and will germinate by bleaching for time t1. Brown indicates seeds that will be killed by bleaching for time t1. Seeds that would germinate with longer bleaching than t1 are indicated by orange. Importantly, there is seen to be a whole class of seeds that cannot be germinated by bleaching because the bleaching time required to release them from dormancy is sufficient to kill them. In other words there are seeds that can never be germinated by bleaching because the bleaching necessary to release the seeds from dormancy exceeds what the seeds can withstand and remain viable. This class is represented by the area in light blue.
In drawing this final graph, I positioned the two normal curves in an arbitrary position along the time axis. The position of each graph no doubt varies from seeds of one species to another and probably even from one seed capsule to another for the same species. Quite possibly there are seeds for which the two curves overlap to such an extent that bleaching will not be able to germinate any significant number of seeds.
Another conclusion from this model is that there exists an optimal bleaching time, that is, the time that maximizes the yellow area in the graph. Much of the lab work in germinating Cyp seeds involves trying to find an approximation to this optimal bleaching time through trial and error. Two or more samples of seeds from a given capsule may be taken and bleached for different periods in order to determine a satisfactory bleaching time. Then the rest of the seeds may be bleached for this "best" time as determined in these trials.
The Actual Effect of Bleaching on Orchid Seed Embryos
In an engrossing study of the effects of bleaching on embryos of the orchid Spathoglottis plicata, Novak and others (2008) found that cells in the embryos that were killed by bleaching in NaOCl fluoresced a distinctive green color when excited by blue light during examination with a digital imaging fluorescence microscope. This autofluorescence permitted a determination of the percentage of the cells in the embryo killed by bleaching for different time intervals in a given concentration of NaOCl and also for bleaching for a given time interval in different concentrations of NaOCl. A remarkable result of this work was the finding that embryos of S. plicata were able to germinate and grow into seedlings even when somewhat more than half the embryo was killed by bleaching! The study also found that bleaching at an appropriate level enhances germination, consistent with effects seen in studies of other orchid species and in my own work with Cyps.
The fact that S. plicata seeds can germinate with a significant number of embryo cells destroyed no doubt arises as a result of the fact these embryos are relatively undifferentiated. Orchid embryo cells in general lack such histodifferentiation, and so it seems probable that embryos of many orchids including Cyps will germinate and grow into healthy seedlings even after significant portions of the cells have been killed by bleaching.
Seed Dormancy Mechanisms
Impermeable Seed Coat
Cyps are temperate climate orchids, and as with many plants in temperate climates, evolution has bestowed them with a mechanism to avoid germination at an inappropriate time: seed dormancy. Were the seeds to germinate in fall, say, the tiny protocorms would need to survive in frozen ground with no prospect of subsequent growth until the coming spring.
The exact mechanism or mechanisms of dormancy in Cyp seeds has long been a subject of speculation. Perhaps the earliest conjecture about the dormancy mechanism is that the outer seed coat or testa is water repellent and thus
 prevents water, essential for germination, from reaching the embryo.
The falsity of this hypothesis can be seen merely by observing
the seeds during bleaching with a good hand lens or low power on a
dissecting microscope. In the seeds of many Cyp species, bubbles
of gas can be seen in the space between the seed coat and the embryo,
the gas being liberated as a result of the chemical reaction between
the bleach and the inner seed coat or the embryo. The bleaching
solution easily enters the opening at the micropylar end of
the outer seed coat (testa) where the seed detached from the placenta. This
opening shows prominently in scanning electron microscope (SEM) photos
of orchid seeds and can also be seen in views with light microscopes at
magnification of 30X or so if the seed is manipulated into the proper
orientation. The photo at the right shows the interior of a
freshly opened C. parviflorum var. pubescens
capsule in which the seeds have already mostly become detached and are
in somewhat randomized orientations. The red circles indicate the
openings in the outer seed coats of some of the seeds. Careful perusal
shows several other seeds with an orientation that allows this opening
to be seen. The seed is indeed sufficiently water repellent that
it can be dispersed in the environment by floating, but if the
seed is trapped underground, soil moisture could very easily enter the
coat through the open end. Thus impermeability of the outer seed coat is not a major hindrance to germination.
prevents water, essential for germination, from reaching the embryo.
The falsity of this hypothesis can be seen merely by observing
the seeds during bleaching with a good hand lens or low power on a
dissecting microscope. In the seeds of many Cyp species, bubbles
of gas can be seen in the space between the seed coat and the embryo,
the gas being liberated as a result of the chemical reaction between
the bleach and the inner seed coat or the embryo. The bleaching
solution easily enters the opening at the micropylar end of
the outer seed coat (testa) where the seed detached from the placenta. This
opening shows prominently in scanning electron microscope (SEM) photos
of orchid seeds and can also be seen in views with light microscopes at
magnification of 30X or so if the seed is manipulated into the proper
orientation. The photo at the right shows the interior of a
freshly opened C. parviflorum var. pubescens
capsule in which the seeds have already mostly become detached and are
in somewhat randomized orientations. The red circles indicate the
openings in the outer seed coats of some of the seeds. Careful perusal
shows several other seeds with an orientation that allows this opening
to be seen. The seed is indeed sufficiently water repellent that
it can be dispersed in the environment by floating, but if the
seed is trapped underground, soil moisture could very easily enter the
coat through the open end. Thus impermeability of the outer seed coat is not a major hindrance to germination.While the outer seed coat itself does not prevent moisture from reaching the embryo, the embryo sac or inner seed coat, called the carapace by Rasmussen (1995), may very well prevent moisture from entering the embryo itself.
Biochemical Dormancy
Biochemical means of maintaining seed dormancy in many plants have been studied intensively over the last several decades, but many details of the mechanisms involved have only partially been elucidated. Extensive research has pretty well established that abscisic acid (ABA) and gibberellic acid (GA) are the primary factors regulating the transition from dormancy to germination. ABA is responsible for the initiation and maintenance of seed dormancy and GA is required for the release from dormancy and the induction of germination. Current studies seek to unravel the mechanisms by which these substances act at genetic and molecular levels. At present, ABA is the only endongenous growth regulator (also known as plant hormone) known to sustain seed dormancy (Liu and others, 2013).
In numerous temperate plants, the technique of cold, moist stratification of seeds has been used for many years to break dormancy in these seeds. Indeed such cold stratification is also sometimes successful in bringing Cypripedium seeds to a state at which germination occurs upon bringing cultures of the seeds up to room temperature. The detailed biochemical pathways by which cold stratification either reduces the ABA content of seeds or increases that of GA have yet to be discovered.
 Because
of its importance in maintaining seed dormancy, I want to mention a
little bit about the chemistry of ABA. The molecular structure is
shown in the figure at left. Despite the somewhat elaborate
configuration of the molecule, its acidic nature is clear from the
presence of the carboxyl group (C(O)OH) shown in pink in the figure.
Because they are proton donors, carboxylic acids are acidic in
the Brønsted-Lowry
sense. Small carboxylic acid molecules are soluble in water, but
solubility decreases with increasing numbers of carbon atoms.
Organic chemistry tells us that carboxylic acids are generally soluble
in weak solutions of strong bases. Indeed while ABA
is only slightly soluble in water, it is quite soluble in 1 N sodium
hydroxide and also in methanol, ethanol, and the industrial solvent dimethyl
sulfoxide (DMSO), the
solubility in these compounds being roughly 20 to 50 mg/mL.
Because
of its importance in maintaining seed dormancy, I want to mention a
little bit about the chemistry of ABA. The molecular structure is
shown in the figure at left. Despite the somewhat elaborate
configuration of the molecule, its acidic nature is clear from the
presence of the carboxyl group (C(O)OH) shown in pink in the figure.
Because they are proton donors, carboxylic acids are acidic in
the Brønsted-Lowry
sense. Small carboxylic acid molecules are soluble in water, but
solubility decreases with increasing numbers of carbon atoms.
Organic chemistry tells us that carboxylic acids are generally soluble
in weak solutions of strong bases. Indeed while ABA
is only slightly soluble in water, it is quite soluble in 1 N sodium
hydroxide and also in methanol, ethanol, and the industrial solvent dimethyl
sulfoxide (DMSO), the
solubility in these compounds being roughly 20 to 50 mg/mL.Several decades of research on seed dormancy and germination have implicated ABA as a major player in dormancy. In particular toward the end of the last century research on the germination of monocot seeds has associated leaching of ABA from the seeds with promoting germination. Such a result was found for barley (Visser and others, 1996) and for wheat (Suzuki and others 2000). This work on barley germination also seemed to show that the ABA-perception site resides outside the embryo, presumably on the embryo surface, and that is the location at which ABA effects its action to inhibit germination.
In a landmark paper for orchid biology, Lee and others (2015) studied ABA during the development of C. formosanum (Taiwan lady's slipper) and demonstrated that 1) ABA is the primary inhibitor of germination of the mature seed; 2) ABA is synthesized internally in embryo cells during early embryo development, and 3) as development continues the ABA migrates from within the cells outward to accumulate in the surface wall of the embryo and the inner seed coat (carapace) and to some extent in the outer seed coat. The distribution of ABA in the mature seed was rendered visible both by immuno-histochemical staining and by immuno-gold labelling. The photos in this paper showing ABA distribution in the seed by both the fluorescence of the immuno-labeled ABA and electron microscopy of the gold-tagged ABA molecules are extremely impressive! I encourage the serious Cyp propagator to read this paper, or at the least, peruse the remarkable photos. Clearly the location of the ABA in the seed coats and on the embryo surface makes it possible to remove the inhibitor by bleaching without killing too many embryo cells.
Application to Cyp Seed Germination
Knowledge that ABA is indeed the key inhibitor of germination and the information about where the ABA is located in the mature seed provide the basis for understanding the effectiveness of the bleaching process. Commercial bleach is not simply a strong solution of sodium hypochlorite, NaOCl. The Clorox website lists the ingredients as: water, sodium hypochlorite, sodium chloride, sodium carbonate, sodium chlorate, sodium hydroxide, and sodium polyacrylate; the sodium hydroxide is used to control the pH.
Because Clorox contains NaOH as well as NaOCl, the effect of the bleach on orchid seeds is twofold: 1) The NaOCl oxidizes compounds in the carapace to cause its weakening through corrosion thus allowing the bleach access to ABA on the embryo surface, and 2) the NaOH in the bleach serves to dissolve the ABA as the bleach penetrates the carapace. Perhaps the NaOCl also attacks ABA. This picture of the bleaching process can also explain differences in response of Cyp seeds to the presoak in NaOH or KOH. Those seeds with relatively thin or permeable carapace such as C. macranthos see enhanced germination when the presoak is followed by relatively short bleaching, while seeds with heavy or impermeable carapace, especially C. arietinum, show little enhancement of germination with the presoak and still require lengthy bleaching for germination.
---------------------------------------------------------------------------
References
Anderson AB. 1989. Asymbiotic germination of seeds of some North American orchids. Pages 75-86 in North American Terrestrial Orchid Propagation and Production Conference Proceedings 1989. Chadds Ford, Pennsylvania.
Lee YI, Chung MC, Yeung EC, Lee N. 2015. Dynamic distribution and the role of abscisic acid during seed development of a lady's slipper orchid, Cypripedium formosanum. Annals of Botany 116: 403-411.
Liu X, Zhang H, Zhao Y, Feng Z, Li Q, Yang HQ, Luan S, Li J, He ZH. 2013. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proceedings of the National Academy of Sciences 110: 15485-15490.
Novak SD, Pardiwala RS, Gray BL. 2008. A study of NaOCl-induced necrosis indicates that only half of the embryo is required for seedling establishment in Spathoglottis plicata. Lindleyana 21: 32-38.
Rasmussen HN. 1995. Terrestrial orchids--from seed to mycotrophic plant. Cambridge: Cambridge University Press.
Suzuki T, Matsuura T, Kawakami, N, Noda K. 2000. Accumulation and leakage of abscisic acid during embryo development and seed dormancy in wheat. Plant Growth Regulation 30: 253-260
Van Waes JM, Debergh PC. 1986. In vitro germination of some Western European orchids. Physiologia Plantarum 67: 253-261.
Visser K, Vissers APA, Çağirgan MI, Kijne JW, Wang M. 1996. Rapid germination of a barley mutant is correlated with a rapid turnover of abscisic acid outside the embryo. Plant Physiology 111: 1127-1133.